The success rate from first candidate to market launch is less than 1%, requiring approximately 22 hits for every drug launched. Within CNS/Neurological conditions specifically, the overall likelihood of approval (LOA) once a drug enters Phase I clinical trials is less than 12%.
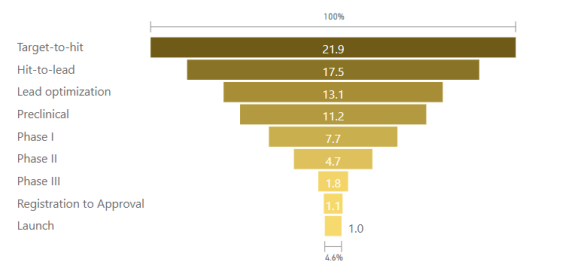
Source: Pharmaceutical Probability of Success, Alacrita Consulting, 2018
Much of the risk can be linked to the inability to successfully translate preclinical data to the clinical situation. This holds true for both safety and efficacy studies. So what can drug developers do to increase the translatability of their preclinical data and better predict in-human efficacy?
1) Utilize models and approaches which increase the probability of success in predicting in-human efficacy.
Disease induction, species selection, and clinical measurements are certainly important considerations in producing data that can predict in-human efficacy. Nearly every therapeutic area is represented by several different in vivo models that aim to reflect the human condition. Understanding the mechanism of disease produced in any given model plays a role in quality preclinical study design.
Furthermore, while rodent models are highly useful in preclinical studies, the ability to assess lead candidates in larger animal models such as the pig lends to even greater translatability. Pigs share many similarities with the human as it relates to the cardiovascular system, central nervous, immune, and digestive system as well as skin innervation. There is also higher correlation between contractile, metabolic, and morphological features in skeletal muscle of humans and pigs.
Wondering how your preclinical program could benefit from pig translational models? Click here to learn more.
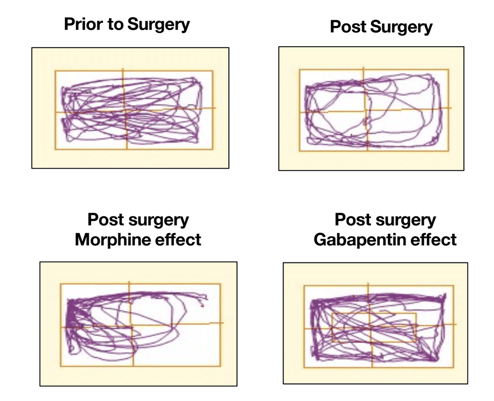 Image: open field assessment for measuring locomotor activity in a pig model of pain
Image: open field assessment for measuring locomotor activity in a pig model of pain
2) Even the best in vivo models have limitations in recapitulating human disease. Drug developers need to incorporate translational approaches through clinically relevant measurements and outcomes.
By using outcomes that are comparable to those measured in patients, researchers can increase the predictability of preclinical studies. As it relates to pain, the clinical outcome is commonly based on patients being able to verbally indicate the degree of pain through rating scales or questionnaires. That however is very different from preclinical animal studies where verbal behavior is not available as a source of measure. The pain diagnosis must then rely on changes in other behaviors that reflect the human response, making the transfer of pain stimulus to behavior analysis important. Researchers that are experts in these behavior responses can bring insight into preclinical study and data analysis.
Going a step further than understanding the typical clinical measures within the study, using biomarkers, staining methods, and electrophysiology can bring important insight, especially to the field of pain and neurodegenerative conditions. For example, intraepidermal nerve fiber (IENF) staining is also a standard clinical tool for diagnosing peripheral neuropathies. Adding this clinically relevant outcome to preclinical studies will enhance the understanding and predictability of that study.
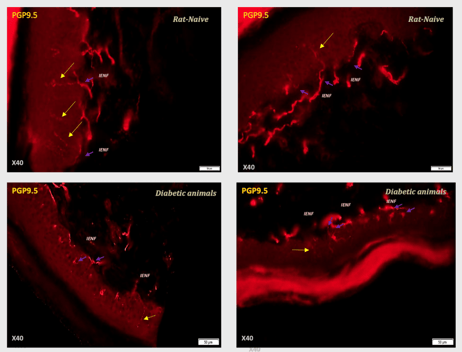
Image: IENF staining in diabetic rats (STZ model of diabetic neuropathy).
Electrophysiology can be conducted to study the functional properties of neurons, which allows researchers to better understand the neurological disorder and the potential of neuroprotective or neurodegenerative therapies. Often times, the data gained through this method can provide insights that may otherwise be missed when relying on measures such as clinical score alone.
To learn more about adding clinically relevant outcomes such as electrophysiology to preclinical studies, click here.
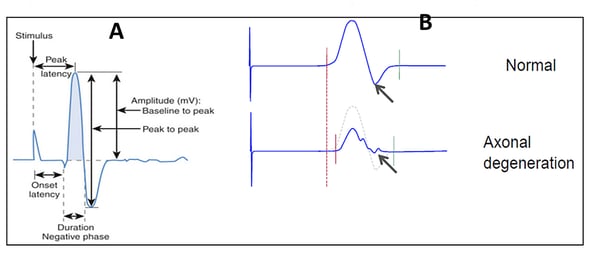
Image: electrophysiology reflecting compound muscle action potentials (cMAPS) from the gastrocnemius muscle for a peripheral nerve injury model in rats
3) Deliver strong preclinical data by designing rigorous and robust efficacy studies considering dosing route, regime, and reproducibility.
This final point seems like it almost doesn’t need to be stated, but surprisingly, the merits of quality, accuracy, and reproducibility can’t be overlooked. The work done every day by drug developers is based on studies that determine the efficacy of a therapy as it enters clinical phases. Studies that involve complex dosing routes and regimes may be necessary in CNS/neurological conditions. Having confidence in the skill of the team in not only inducing the disease condition, but dosing the therapy, measuring the response, and analyzing and interpreting the data goes a long way in being able to make the critical decisions needed to move candidates forward in the development pipeline.
Incorporating these three strategies (among others) into CNS/pain development programs will help provide drug developers with translatable data needed to successfully progress to the clinic. The work you do every day is bringing critical therapies to many in need. If you would like to expand your internal capabilities to a partner with deep expertise in designing studies that achieve all 3 strategies, contact us to discuss your program.





 Image: open field assessment for measuring locomotor activity in a pig model of pain
Image: open field assessment for measuring locomotor activity in a pig model of pain

